Clinical trials

At Quirónsalud, we conduct research to contribute to the development of more effective therapies that improve healthcare and the quality of life of patients. We have approximately 1,000 active clinical trials through which we aim to improve and innovate in the treatment of diseases that require new drugs and techniques, as well as provide our patients with early access to the most innovative treatments.
A clinical trial is a medical investigation that is most often conducted on a medication, although healthcare products and new diagnostic or therapeutic techniques, or those that have never been tested on people for this purpose, are also studied. The objective of clinical trials is to study the effects of an innovative treatment on human beings to determine its efficacy and safety. Therefore, they are essential to understand its behavior before being used in people.

First, participating in a clinical study contributes to the advancement of treatment for many diseases and helps improve the understanding of these conditions. However, it is also an opportunity to access new treatments for individuals who have exhausted all possible therapies for their disease. In fact, for certain patients in whom conventional treatments are ineffective, participating in a trial may be their only option for improvement.

The requirements to participate in a clinical trial depend on the disease being targeted, and they are usually related to it. In addition to these specific conditions, other general conditions must also be met, which are established as minimum criteria and include factors known as inclusion criteria. There are also exclusion criteria, which are reasons that prevent a person from participating in a trial.
In any case, the medical researcher in charge of conducting the clinical trial is always the most qualified person to inform you in detail about whether you meet these criteria.

In some clinical trials, participants are divided into groups, where part of the volunteers receives the new medication, and part receives the standard treatment or a placebo.
The knowledge of the intervention by patients or researchers may influence the evaluation of the response. Depending on the level of knowledge that patients or researchers have, trials can be classified as:
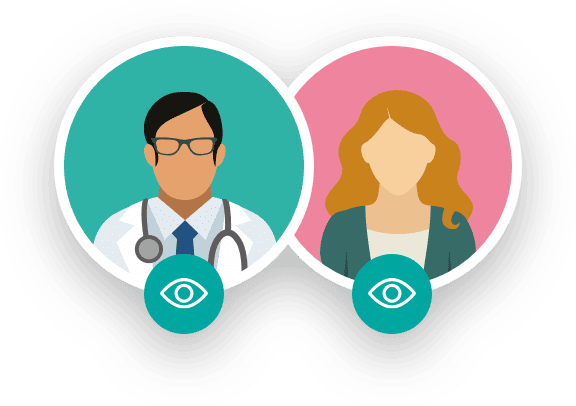
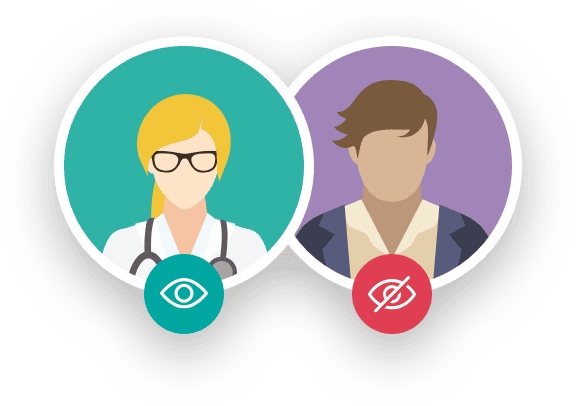
It is essential to clarify that before a medication or healthcare product becomes part of a clinical trial, this research has been approved by an Ethics Committee composed of various healthcare professionals, legal experts, and patient representatives. This committee evaluates whether the benefits that can be obtained from the research results outweigh the risks associated with the proposed actions.
Furthermore, before any clinical trial is conducted, medications or healthcare products undergo a preclinical phase involving animal experimentation, during which all basic safety and toxicity aspects are measured. This information is also submitted for evaluation by the relevant national authorities, such as the Spanish Agency for Medicines and Health Products.
Finally, it is important to highlight that during the clinical trial, all possible measures are taken to ensure the safety of participants. Continuous tests and visits are conducted, including blood tests, physical examinations, diagnostic imaging procedures, and, of course, interviews and phone contact with the medical researcher overseeing the trial.
Despite the advantages that participating in a clinical trial offers to a patient, it must be understood that clinical trials are not without risks, as they are designed to understand the effects of a new treatment. Generally, these risks are limited to minor discomforts, although in very rare cases, complications may arise, which are addressed immediately during the follow-up process.
No, not everyone can participate in a clinical trial. Depending on the type of trial, specialists will require certain characteristics that are typically related to the disease being studied, as well as other factors concerning the general health status and medical history of the individual.
These are specific to each study and are directly related to the disease being studied, the degree of severity of the condition, the treatments previously received, current medication, medical history, and other factors that are disclosed for each individual case.
Yes, participation in a clinical trial is completely voluntary. Therefore, if at any point a patient wishes to withdraw, they may do so freely, without needing to provide a reason or justification.
The duration depends on each trial, but in any case, this information will be provided before the study begins.
A placebo is a substance with no pharmacological activity but that looks identical to the medication being studied in the clinical trial. It is given to a portion of the participants to compare its effects with those of the drug being evaluated. The goal is to rule out therapeutic effects due to the mere feeling of being treated.
As a general rule, this information is only disclosed after the trial has been completed, in order to prevent this knowledge from influencing the response to the treatment.
Quirónsalud has received the mutual recognition granted by the international Transcelerate initiative as a Good Clinical Practice (ICH E6) Trainer.
This recognition implies that Quirónsalud provides continuous training at all its centers for all personnel involved in clinical research on the minimum requirements that must be met according to the international ICH GCP guidelines for conducting clinical trials. This is a quality standard, both ethical and scientific, applicable to the design, conduct, recording, and communication of clinical trials involving human participants.
Compliance with this standard provides a public guarantee of the protection of the rights, safety, and well-being of individuals participating in clinical trials, in accordance with the principles of the Declaration of Helsinki. It also guarantees the credibility of the data collected during the clinical trial.